Nrf2-GPX4信号通路与冠心病患者相关性研究
摘 要:目的:通过检测血清Nrf2、GPX4活性水平,分析其与冠心病(coronary heart disease,CHD)以及冠脉病变严重程度的相关性,探讨其信号通路介导的铁死亡途径在CHD病情进展中的作用。方法:选取540例疑诊CHD患者,行冠状动脉造影检查,其中360例病人明确诊断为CHD,冠状动脉造影无异常180例为对照组(CON组)。通过ELISA检测CHD患者血清中Nrf2和GPX4活性水平,统计分析在有无CHD血清中Nrf2和GPX4活性差异,Western blot 检测CON组和CHD组(各90例)外周血单核细胞(peripheral blood mononuclear cells,PBMCS)Nrf2蛋白的表达,分析其信号通路在CHD患者中的表达意义。结果:CHD组中Nrf2、GPX4活性水平均较对照组降低(P<0.05),且Westernblot检测两组患者PBMCs中Nrf2的表达水平:CHD组Nrf2(0.25±0.05)蛋白表达水平较CON组(0.87±0.16)是呈下调趋势(P<0.05),表明Nrf2蛋白表达水平在CHD患者是低表达的。Pearson相关性分析显示:血清Nrf2、GPX4水平与Gensini评分负相关(Nrf2:r=-0.347,P<0.001;GPX4:r=-0.423,P=0.001)。Nrf2、GPX4与TG负相关(Nrf2:r=-0.284,P<0.001;GPX4:r=-0.275,P=0.001),Nrf2和GPX4水平与 LDL 呈负相关(Nrf2:r=-0.418,P<0.05;GPX4:r=-0.426,P<0.05),Nrf2和GPX4水平与HDL呈正相关(Nrf2:r=0.318,P<0.05;GPX4:r=0.428,P<0.05),Nrf2与GPX4呈正相关(r=0.456,P<0.01)。结论:Nrf2-GPX4信号通路介导的铁死亡途径与冠脉病变程度及CHD的发病机制有着密切关系,且其作用机制可能与Nrf2-GPX4信号通路表达下调有关。
关键词:冠状动脉疾病;病变狭窄程度; Nrf2; GPX4;
Correlation between Nrf2-GPX4 signaling pathway and patients with coronary
heart disease
XING Bu-dian LI Hui QIANG Tian-tian WEI Ting LU Yuan-yuan KANG Pin-fang
ZHANG Ning-ru
Department of Cardiology, First ffliated Hospital of Bengbu Medical College
Basic
and Clinical Key Laboratory of Cardiovascular and Cerebrovascular Diseases, First
filiated Hospital of Bengbu Medical College Department of Ophthalmology, First
Affliated Hospital of Bengbu Medical College Bengbu Third People's Hospital Afliated
to Bengbu Medical College
Abstract:Objective: To analyze the correlation between serum Nrf2 and GPX4 activity levels with coronary heart disease (CHD) and the severity of coronary artery disease, and to explore the role of ferroptosis mediated by its signaling pathway in the progression of CHD. Methods: A total of 540 patients suspected of CHD were selected for coronary angiography, of which 360 patients were diagnosed with CHD. The activity levels of Nrf2 and GPX4 in the serum of CHD patients were detected by ELISA, and the differences in the activities of Nrf2 and GPX4 in the presence or absence of CHD were statistically analyzed. Western blot detection of Nrf2 protein expression of peripheral blood mononuclear cells (PBMCS) in CON and CHD groups (90 cases each).The expression and significance of its signaling pathway in CHD patients were analyzed. Results: The activity levels of Nrf2 and GPX4 in the CHD group were lower than those in the control group (P<0.05), and the expression levels of Nrf2 in PBMCs of the two groups were detected by Western blot: The protein expression level of Nrf2 in CHD group (0.25±0.05) was down-regulated compared with CON group (0.87±0.16)(P<0.05),indicating that Nrf2 protein expression level is low in CHD patients. Pearson correlation analysis showed that serum Nrf2 and GPX4 levels were negatively correlated with Gensini score (Nrf2: r=-0.347, P<0.001; GPX4: r=-0.423, P=0.001). Nrf2 and GPX4 were negatively correlated with TG (Nrf2: r=-0.284, P<0.001; GPX4: r=-0.275, P=0.001), and Nrf2 and GPX4 levels were negatively correlated with LDL (Nrf2: r=-0.418, P<0.001) 0.05; GPX4: r=-0.426, P<0.05), Nrf2 and GPX4 levels were positively correlated with HDL (Nrf2: r=0.318, P<0.05; GPX4: r=0.428, P<0.05), Nrf2 was positively correlated with GPX4 ( r=0.456, P<0.01). Conclusion: The ferroptosis pathway mediated by the Nrf2-GPX4 signaling pathway is closely related to the degree of coronary artery disease and the pathogenesis of CHD, and its mechanism may be related to the down-regulation of the Nrf2-GPX4 signaling pathway.
Keyword:Coronary artery disease; Degree of stenosis; Nrf2; GPX4;
冠心病(coronary heart disease,CHD)是主要是由于冠状动脉狭窄或者供血不足,继而引发心肌供氧和血供应不足、心肌血管耗氧急剧增加,最终导致心肌坏死[1,2,3]。流行病学调查结果表明,CHD严重危害人类生命健康,并呈现年轻化、发病率逐年上升的趋势[4]。
谷胱甘肽过氧化物酶4(glutathione peroxidase4,GPX4)是一种重要的抗氧化酶,能够将脂质ROS还原为无毒的脂质醇,减少脂质过氧化,有效预防了细胞的氧化应激,从而抑制了铁死亡进程[5]。近期研究[6]发现,GPX4介导的铁死亡与CHD有着密切的联系。Nrf2抗氧化剂是细胞氧化应激和铁死亡重要的信号通路[7,8]。近年来相关研究[9,10]发现,激活Nrf2可通过促进GPX4的表达进而抑制铁死亡。而目前Nrf2-GPX4信号通路介导的铁死亡在CHD 中的发病机制尚不明确。本研究拟通过检测Nrf2及GPX4水平,分析其与冠状动脉狭窄程度不同CHD病人的关系,为CHD诊断治疗提供新依据。
1 资料与方法
1.1材料试剂
Nrf2抗体(货号:12721S;产地:美国CST)、GAPDH抗体(货号:5174;产地:美国CST);ELISA检测试剂盒:HumanNrf2(上海羽朵)、HumanGPX4(上海羽朵)
1.2 研究对象
收集2020年6月至2022年6月我院540例疑诊CHD患者,平均年龄(64.52±9.66)岁,根据CHD诊断指南[11],以任意一支主要冠脉狭窄≥50%即可诊断为CHD。其中,360例明确诊断为CHD,冠状动脉造影无异常180例患者纳入CON组。入选患者均签署知情同意书。所有入选患者均排除既往有心肌炎、心肌病、恶性肿瘤类疾病、风湿免疫系统疾病、内分泌及代谢性疾病及感染性疾病[12]。入选患者均签署知情同意书,研究方案获得本院伦理委员会批准( 2019KY023)。
1.3方法
1.3.1标本采集
所有患者入院后空腹过夜后,抽取外周静脉血10 mL,置于肝素抗凝管中,3000 r/min离心10 min后吸取上清,并于-80 ℃下冷冻保存备用。
1.3.2 ELISA检测患者血清中Nrf2、GPX4水平
采用 HumanNrf2、GPX4(上海羽朵)检测受试者血清中Nrf2、GPX4的表达水平,严格按说明书进行操作。
1.3.3 Western blot检测Nrf2-GPX4信号通路在CHD组和CON组中的Nrf2蛋白的表达
提取PBMCS中总蛋白,依据BCA蛋白定量法,测定各组蛋白浓度,配置10%SDS-PAGE电泳,PVDF膜转膜,抗体稀释比:抗Nrf2兔单克隆抗体1∶1 000、抗GAPDH兔单克隆抗体1∶10 000;一抗二抗孵育;洗膜后ECL发光液曝光,成像系统获取图像[13]。
1.3.4统计学方法
组间比较采用t检验、卡方检验,应用Pearson分析法分析指标间的相关性,以上数据采用SPSS26.0软件分析。
2. 结果
2.1受试者一般资料和生化指标分析
与CON组比较,CHD组年龄、吸烟史、高血压病史、糖尿病史、TG、LDL均是升高的;而HDL却是降低的(P均<0.05);而两组间性别比、BMI、WBC、AST、ALT、TC、和Scr无明显差异(P> 0.05),结果见表 1。
表1 CON组和CHD组的一般资料和生化指标比较(`x±s)
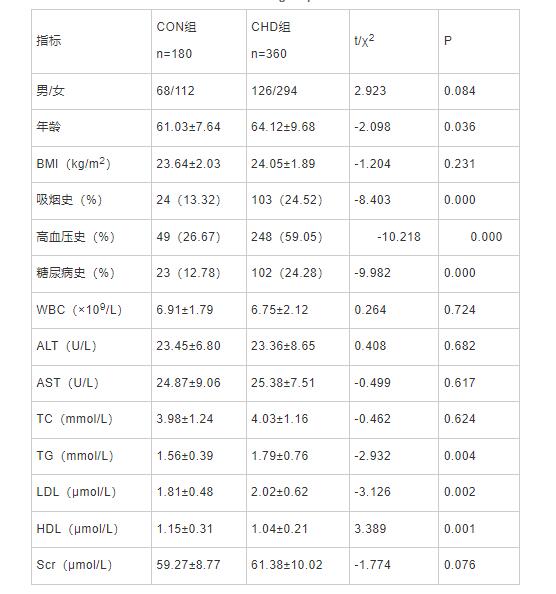
Tab 1 Comparison of general data and biochemical indexes between CON group and CHD group
注:WBC:白细胞计数;BMI:体重指数;ALT:谷丙转氨酶;AST:谷草转氨酶;TC:总胆固醇;TG:甘油三酯;HDL:高密度脂蛋白;LDL:低密度脂蛋白;Scr:血肌酐;
2.2 CON组和CHD组受试者Nrf2、GPX4以及Gensini评分
与CON组比较,CHD组血清Nrf2、GPX4活性水平均是降低的;而Gensini评分却是明显增大的(P均<0.05)结果见表2。
表2 CON组和CHD组受试者Nrf2、GPX4以及Gensini评分比较
Tab 2 Comparison of Nrf2, GPX4 and Gensini scores between CON group and CHD group
2.3 CON组和CHD组受试者(各90例)PBMCS中Nrf2蛋白表达水平
CHD组Nrf2(0.25±0.05)蛋白表达水平较CON组(0.87±0.16)是呈下调趋势的(P<0.05),见图A,图B。
图A CON组和CHD组患者PBMCs中蛋白条带
图B 两组患者PBMCs中蛋白表达量相对大小
2.4 Nrf2、GPX4与Gensini 评分以及血液指标的相关性
经Pearson相关性分析:血清Nrf2、GPX4水平与Gensini评分负相关(Nrf2:r=-0.347,P<0.001;GPX4:r=-0.423,P=0.001);Nrf2、GPX4与TG负相关(Nrf2:r=-0.284,P<0.001;GPX4:r=-0.275,P=0.001);Nrf2和GPX4水平与 LDL 呈负相关(Nrf2:r=-0.418,P<0.05;GPX4:r=-0.426,P<0.05);Nrf2和GPX4水平与HDL呈正相关(Nrf2:r=0.318,P<0.05;GPX4:r=0.428,P<0.05);Nrf2与GPX4呈正相关(r=0.456,P<0.01),结果见表3。
表3 血清Nrf2及GPX4活性水平与 Gensini评分以及血液指标的相关性分析
Tab 3 Correlation analysis of serum Nrf2 and GPX4 activity levels with Gensini scores and blood indexes
3 讨论:
CHD是临床上最常见、最主要的疾病,主要由冠脉粥样硬化介导的管腔狭窄或堵塞所导致,且随着冠脉病变的加重,从而更容易造成冠状动脉的狭窄或者供血不足,最终引起心肌功能障碍[14,15]。迄今为止,CHD发病机制尚未明确。CHD高致残率与致死率的特点,给患者的生命安全与生活质量造成影响。因而,深入地研究CHD发生机理,有助于CHD的诊疗及早期预防。
研究表明,血脂异常为CHD发生的一种重要危险因素[16]。已有相关研究[17,18]指出,高TG血症、高LDL血症、低HDL血症与CHD的发生、发展密切相关,且通过改善患者血脂水平,有利于缓解CHD症状,促进病情转归。在本次研究中,CHD组TG、LDL明显高于CON组,而HDL水平却是降低的,考虑机制可能与血脂异常致动脉粥样硬化有关。
铁死亡是非凋亡的细胞死亡的一种形式,依赖于铁元素的脂质活性氧( ROS) 的积累。脂质ROS在细胞内的不断堆积,引发细胞内的氧化应激反应,逐步诱发细胞的铁死亡[19]。近年来多项研究表明[20,21],铁死亡与CHD密切相关。
GPX4是一种选择性中和脂质氢过氧化物的硒酶,被认为是铁死亡的重要内源性调节剂。有研究表明[22],抑制GPX4的活性,其催化的还原反应不能代谢过氧化脂质,脂质过氧化产生的Fe2+和ROS的不断积累最终导致铁死亡。鉴于GPX4在铁死亡进程中的起着重要作用,其成为铁死亡调控因子的研究热门。Meng等[23]在糖尿病动脉粥样化小鼠模型中观察到,GPX4活性降低引起脂质过氧化,继而引起铁死亡,促进了动脉粥样硬化的发展,而铁死亡抑制剂通过增加GPX4活性,减少脂质活性氧的生成,进而减缓了动脉粥样硬化的发展,该现象表明GPX4活性可能与可能与心肌缺血性疾病CHD有潜在联系。FENG等[24]研究发现,铁死亡抑制剂通过增加GPX4活性,减少了脂质活性氧的生成,继而保护大鼠心脏减轻缺血性损伤。此外,Li等[25]通过实验发现,恢复GPX4活性水平,能够抑制铁死亡,继而可保护小鼠心肌免受缺血再灌注损伤。近期临床实验研究[6]发现,ACS患者GPX4活性水平明显低于非ACS病人。这与本此研究结果相类似,本研究发现相较于CON组,CHD患者GPX4活性水平是降低的,提示GPX4可能参与了CHD的发生、发展。
Nrf2作为铁死亡主要调节因子,其不仅是氧化应激反应和铁死亡的参与者,同时也是氧化应激反应和铁死亡过程的调控者。Nrf2调控着与铁死亡相关的酶和蛋白质如GPX4[9]。Yu等[26]通过研究发现,抑制Nrf2可通过下调GPX4表达,增加了铁死亡,进而促进了小鼠动脉粥样硬化的形成,而铁死亡抑制剂可通过激活Nrf2,增加GPX4表达,使铁死亡进程受抑制,继而抑制了小鼠动脉粥样硬化的形成,该现象表明Nrf2-GPX4信号通路介导的铁死亡可能与CHD有潜在的联系。据报道,柚皮素可以通过调节大鼠体内Nrf2-GPX4信号通路,抑制铁死亡,减轻心肌缺血性损伤[27]。陈玉等[10]通过实验观察到,激活Nrf2-GPX4信号通路,能够抑制铁死亡,减轻了大鼠心肌缺血性损伤。本研究结果显示,较CON组相比,CHD组患者血清Nrf2活性水平是明显降低的,此外,本研究完善了PBMCS中Nrf2蛋白浓度的测定,WB蛋白浓度变化趋势与ELISA结果一致,缪莹[28]也报道了类似结果。Pearson 相关分析显示,CHD患者血清Nrf2与GPX4呈正相关关系,而其作用机制可能通过氧化应激和铁死亡相互联系,从而可能参与了CHD的发生、发展。
Gensini评分是评估冠脉狭窄严重程度的有效方法,分值越高,提示冠脉狭窄越严重[29]。本次研究发现,Nrf2和GPX4与Gensini评分均呈负相关,这一结果提示Nrf2-GPX4信号通路介导的铁死亡与CHD病变程度有关,对于评价CHD患者严重性具有一定的积极意义。
综上所述,与CON组比较,CHD组血清Nrf2与GPX4活性水平是降低的,Nrf2与TG、LDL、以及GPX4水平之间呈正相关,Nrf2和GPX4分别与HDL呈负相关,且Nrf2和GPX4与Gensini评分均呈负相关。因此,Nrf2-GPX4信号通路介导的铁死亡对于判断冠脉病变程度及CHD的发病机制具有一定的参考价值。本研究存在以下不足:纳入样本量少、数据误差不可避免,因此,本文结果还需大规模多中心研究进一步验证。
作者贡献度说明:
邢布点:数据分析、文章撰写工作;强甜甜、魏婷、卢园园:收集数据,负责相关实验操作;李辉、康品方、张宁汝:设计实验,指导写作。本研究作者声明任何与本文有关的利益冲突均不存在。
参考文献
[1] Wu GS, Li HK, Zhang WD. Metabolomics and its application in the treatment of coronary heart disease with traditional Chinese medicine[J].Chin J Nat Med,2019,17(5):321-330.
[2] Zheng J, Zhang S, Wang T. Expression of Notch-1 and nuclear factor-κB signal pathway in myocardial cells of coronary heart disease rats[J]. Exp Ther Med,2019,17(3):1587-1592.
[3] Szabó MR, Pipicz M, Sárközy M, et al.Diet-Induced Hypercholesterolemia Leads to Cardiac Dysfunction and Alterations in the Myocardial Proteome[J].Int J Mol Sci,2022,23(13):7387.
[4] Katta N, Loethen T, Lavie CJ, et al. Obesity and Coronary Heart Disease: Epidemiology, Pathology, and Coronary Artery Imaging[J].Curr Probl Cardiol,2021,46(3):100655.
[5] Kajarabille N, Latunde-Dada GO. Programmed Cell-Death by Ferroptosis: Antioxidants as Mitigators[J].Int J Mol Sci,2019,20(19):4968.
[6] 包炳蔚,丁丝雨,季春斐等.GPX4水平与急性冠状动脉综合征病人临床特征、危险分层及其预后相关性研究[J].蚌埠医学院学报,2022,47(7):841-846.
[7] Kuang Y, Zhang Y, Xiao Z, et al. Protective effect of dimethyl fumarate on oxidative damage and signaling in cardiomyocytes[J].Mol Med Rep,2020,22(4):2783-2790.
[8] Ravingerová T, Kindernay L, Barteková M, et al. The Molecular Mechanisms of Iron Metabolism and Its Role in Cardiac Dysfunction and Cardioprotection[J].Int J Mol Sci, 2020,21(21):7889.
[9] Scuderi SA, Ardizzone A, Paterniti I, et al. Antioxidant and Anti-inflammatory Effect of Nrf2 Inducer Dimethyl Fumarate in Neurodegenerative Diseases[J].Antioxidants (Basel). 2020;9(7):630.
[10] 陈玉,苏建军,韩允等.富马酸二甲酯调控Nrf2-GPX4介导的铁死亡途径对大鼠心肌缺血/再灌注损伤的保护作用研究[J].天津医药,2022,50(6):601-607.
[11] 王康,智丽霞,石姣姣等.冠心病患者血浆白脂素和分泌型卷曲相关蛋白5与冠状动脉病变程度的相关性[J].临床心血管病杂志,2022,38(6):455-460.
[12] 康品方,戎李,孙硕等.血清ALDH2和NLRP3活性水平在冠心病不同血管病变程度中的变化[J].蚌埠医学院学报,2020,45(7):854-858.
[13] 冷俊杰,李辉,姚卓亚等.活化转录因子4在心房颤动患者血浆和外周血单核细胞中的表达及与预后的相关性[J].临床心血管病杂志,2022,38(1):63-67.
[14] Miao L, Yin RX, Huang F, et al.Integrated analysis of gene expression changes associated with coronary artery disease[J].Lipids Health Dis,2019,18(1):92.
[15] Capodanno D, Angiolillo DJ. Antithrombotic Therapy for Atherosclerotic Cardiovascular Disease Risk Mitigation in Patients With Coronary Artery Disease and Diabetes Mellitus[J]. Circulation,2020,142(22):2172-2188.
[16] Jawad M, Vamos EP, Najim M, et al. Impact of armed conflict on cardiovascular disease risk: a systematic review[J].Heart,2019,105(18):1388-1394.
[17] 秦斌,王飞,张文静.阿托伐他汀联合氯吡格雷在冠心病心绞痛治疗中对血脂、血液流变学及心功能影响[J].临床军医杂志,2021,49(1):92-94
[18] Dong J, Yang S, Zhuang Q, et al. The Associations of Lipid Profiles With Cardiovascular Diseases and Death in a 10-Year Prospective Cohort Study[J].Front Cardiovasc Med. 2021,8:745539.
[19] Sorrenti V, Vanella L, Platania CBM, et al. Novel Heme Oxygenase-1 (HO-1) Inducers Based on Dimethyl Fumarate Structure[J].Int J Mol Sci. 2020;21(24):9541.
[20] Li S, Zhang X. Iron in Cardiovascular Disease: Challenges and Potentials[J].Front Cardiovasc Med,2021,8:707138.
[21] Leng Y, Luo X, Yu J, et al. Ferroptosis: A Potential Target in Cardiovascular Disease[J].Front Cell Dev Biol,2022,9:813668.
[22] Ni J, Chen K, Zhang J,et al. Inhibition of GPX4 or mTOR overcomes resistance to Lapatinib via promoting ferroptosis in NSCLC cells[J].Biochem Biophys Res Commun,2021,567,154-160.
[23] Meng Z, Liang H, Zhao J, et al. HMOX1 upregulation promotes ferroptosis in diabetic atherosclerosis[J].Life Sci,2021,284:119935.
[24] FENG YC,ALIAGAN ACI,TOMBO NC,et al.Inhioition of ferroptosis by liproxstatin-1 compound protects the myocardium against ischemia /reperfusion injury by decreasing VDAC1 levels and increasing in GPX4 activity[J].Faseb J,2019,520(3):606.
[25] Li W, Li W, Leng Y, et al. Ferroptosis Is Involved in Diabetes Myocardial Ischemia/Reperfusion Injury Through Endoplasmic Reticulum Stress[J].DNA Cell Biol, 2020,39(2):210-225.
[26] Yu W, Liu W, Xie D, et al. High Level of Uric Acid Promotes Atherosclerosis by Targeting NRF2-Mediated Autophagy Dysfunction and Ferroptosis[J].Oxid Med Cell Longev,2022,2022:9304383.
[27] Xu S, Wu B, Zhong B, et al. Naringenin alleviates myocardial ischemia/reperfusion injury by regulating the nuclear factor-erythroid factor 2-related factor 2 (Nrf2) /System xc-/ glutathione peroxidase 4 (GPX4) axis to inhibit ferroptosis[J].Bioengineered,2021,12(2):10924-10934.
[28] 缪莹. 泸州地区40岁以上2型糖尿病患者发生冠心病的影响因素及Nrf2、IRS-2与T2DM合并冠心病的相关性研究[D].西南医科大学,2018.
[29] Wierzbowska-Drabik K, Picano E, Simiera M, et al. A head-to-head comparison of wall motion score index, force, strain, and ejection fraction for the prediction of SYNTAX and Gensini coronary scores by dobutamine stress echocardiography[J].Kardiol Pol,2020,78(7-8):715-724.



